EMROK Efficacy results (Phase III trial)
Aim
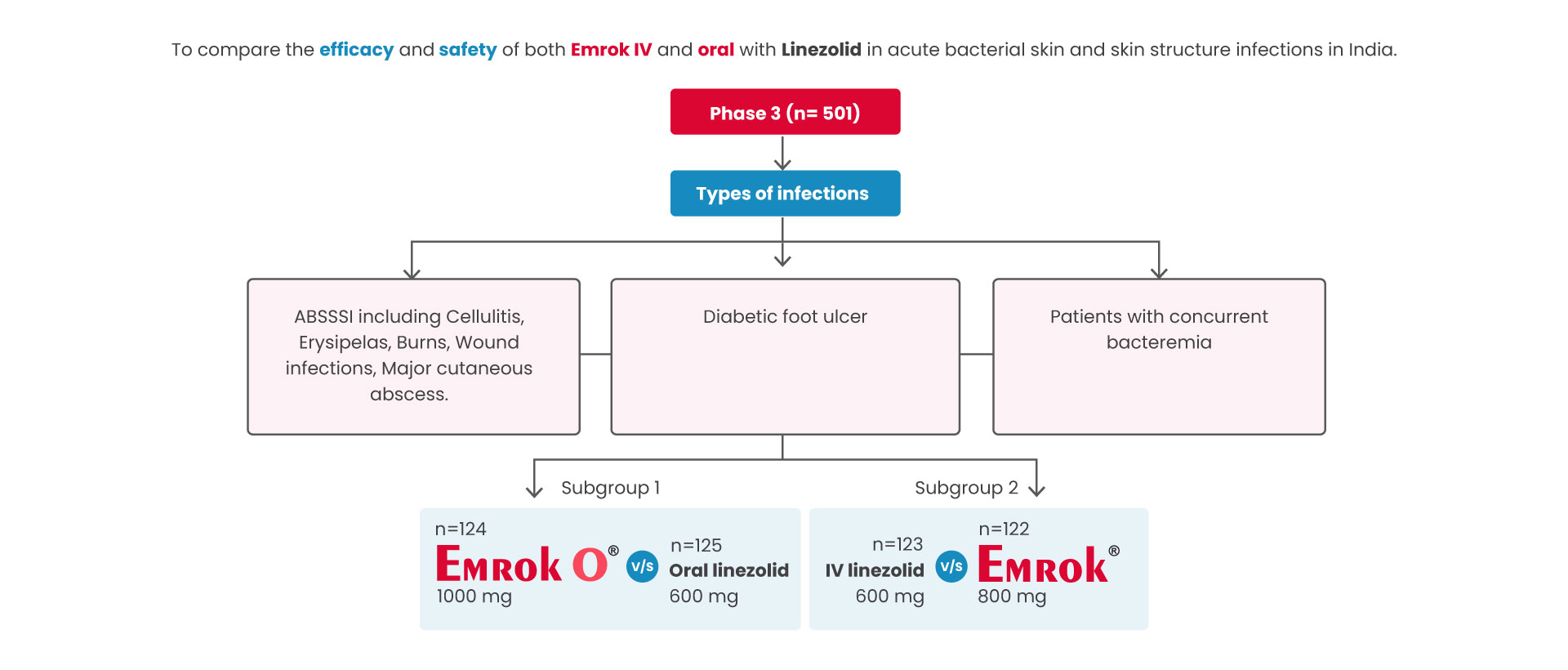
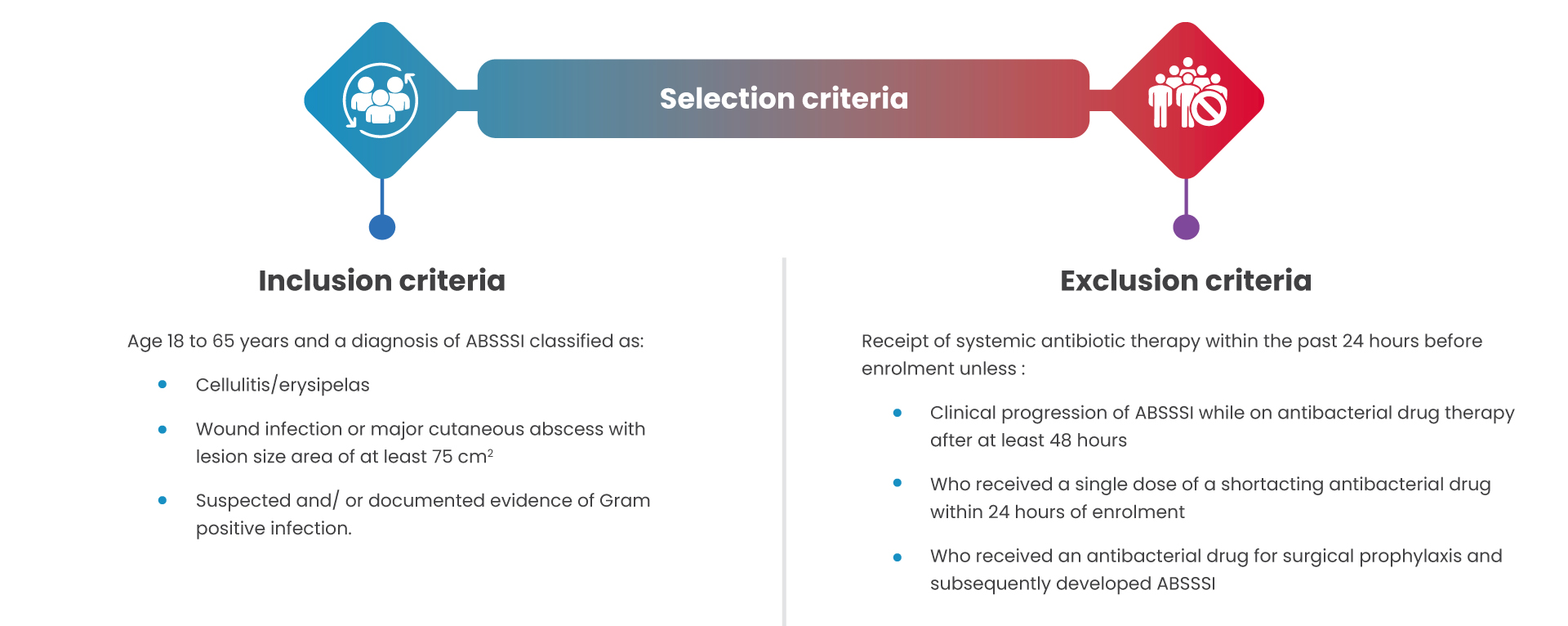
Trial design
Primary outcome
Primary outcome was defined as Overall clinical response at the TOC Visit in the modified intent-to-treat (mITT) population separately for the Oral and IV subgroups.
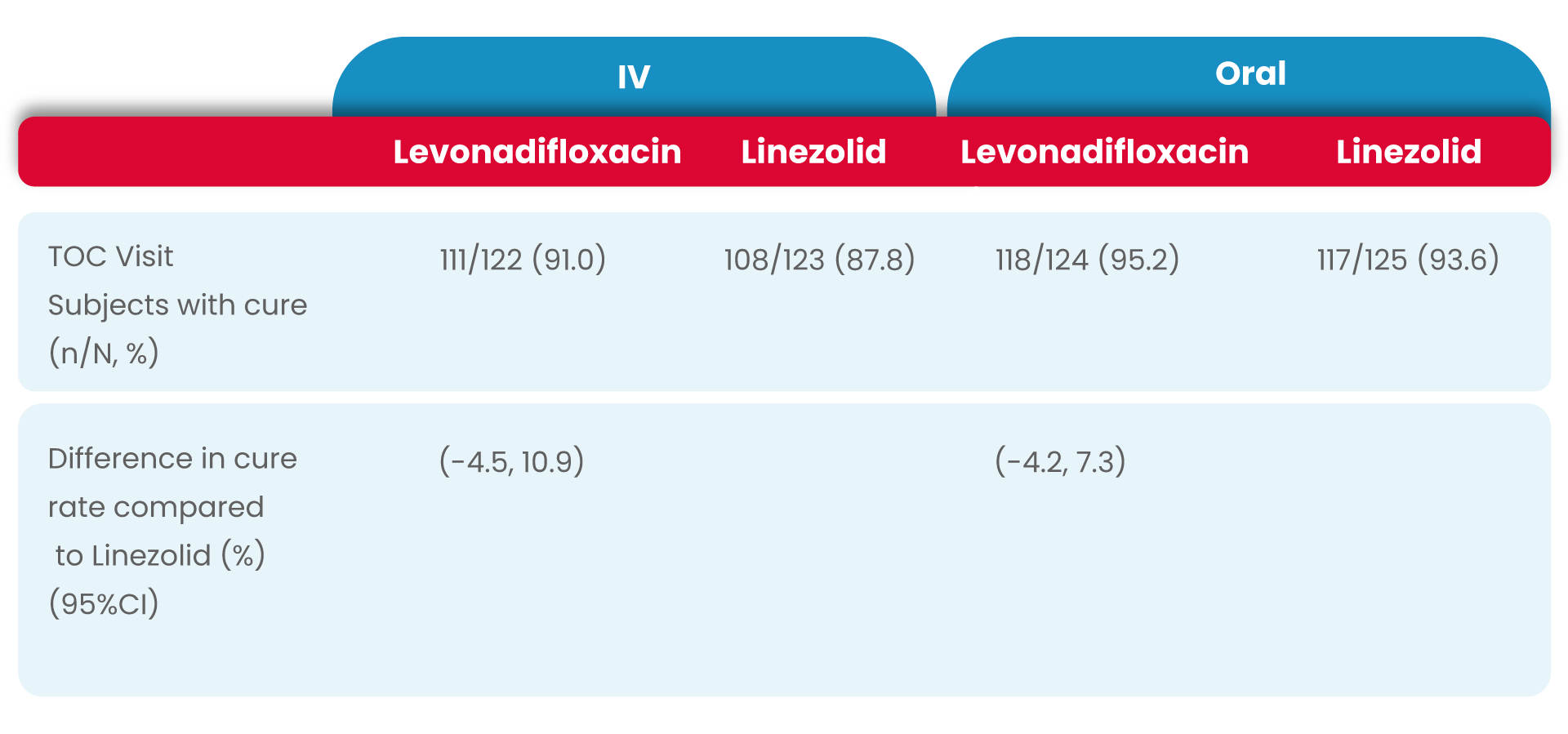
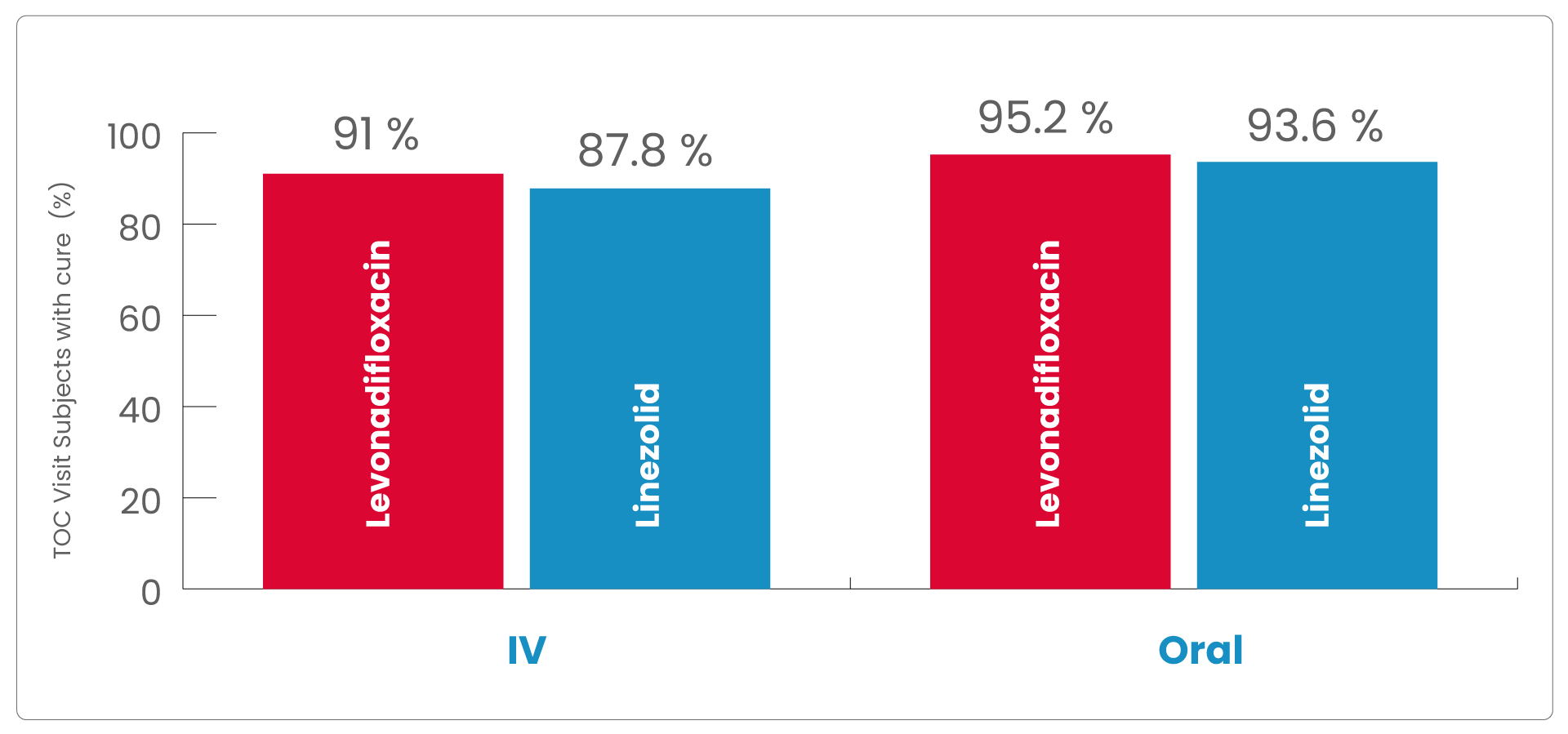
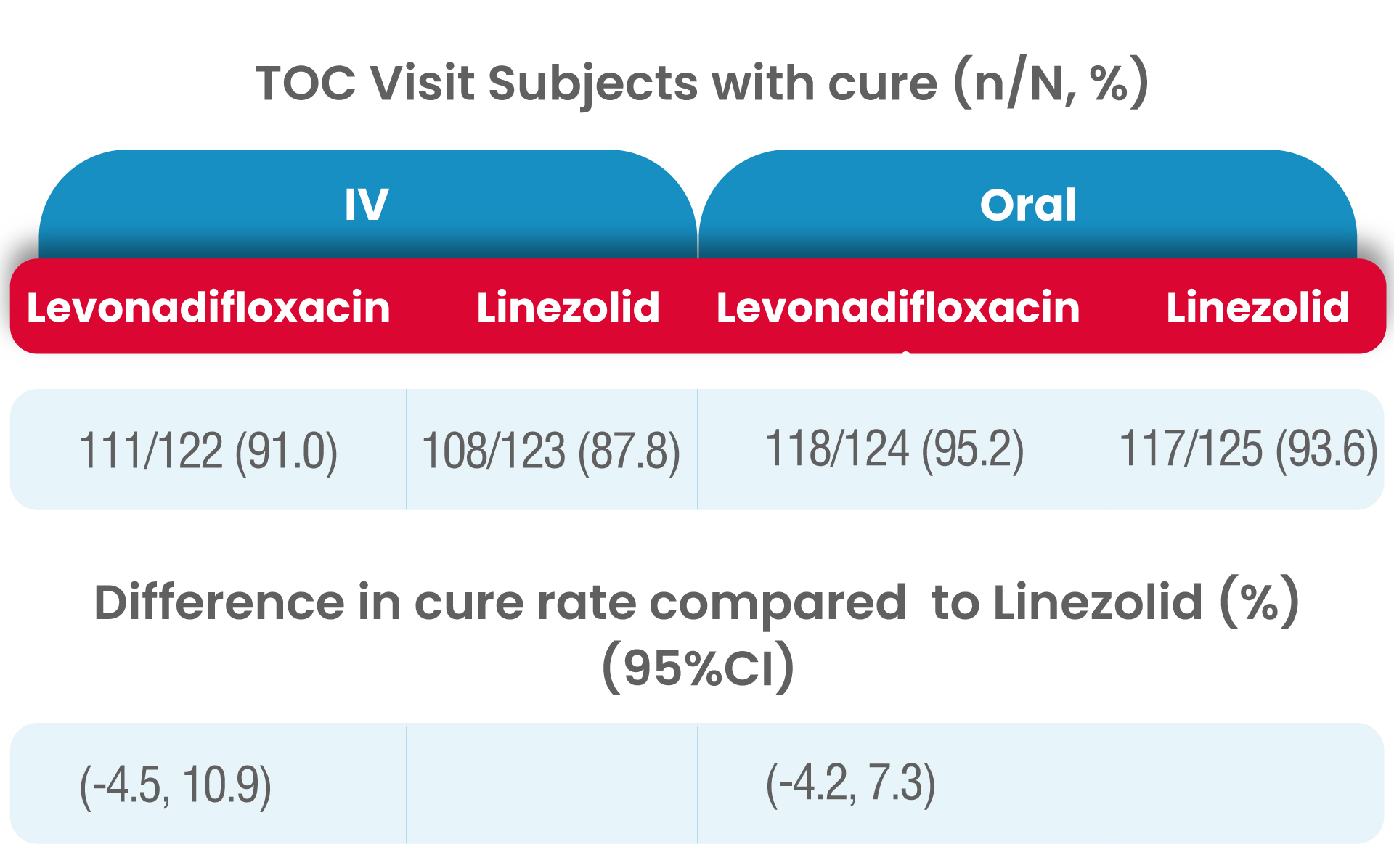

The clinical cure rates observed at the TOC in the mITT (modified Intent to treat) populations for levonadifloxacin was numerically higher compared to linezolid in the IV sub-group [(91.0% verses 87.8%); treatment difference of 3.2% (95%CI, -4.5 to 10.9)] and in the oral sub-group (95.2% versus 93.6%); treatment difference of 1.6 % [95%CI, -4.2 to 7.3]).
As the lowerbound of the 95% CI around the treatment difference was greater than -15% for both subgroups, the primary objective of the study was met.
Therefore, both IV levonadifloxacin and oral levonadifloxacin were non-inferior to IV linezolid and oral linezolid, respectively.
Secondary Outcome
was defined as Clinical response at Visit 3 in the mITT population, the overall clinical response at the EOT Visit in the mITT and the clinically evaluable intent-to-treat (CE-ITT) populations, the overall clinical response at the TOC Visit in the CE-ITT population, and the microbiological response at the EOT and TOC Visits in the microbiological intent-to-treat (micro-ITT) and the microbiologically evaluable intent-to-treat (ME-ITT) populations.
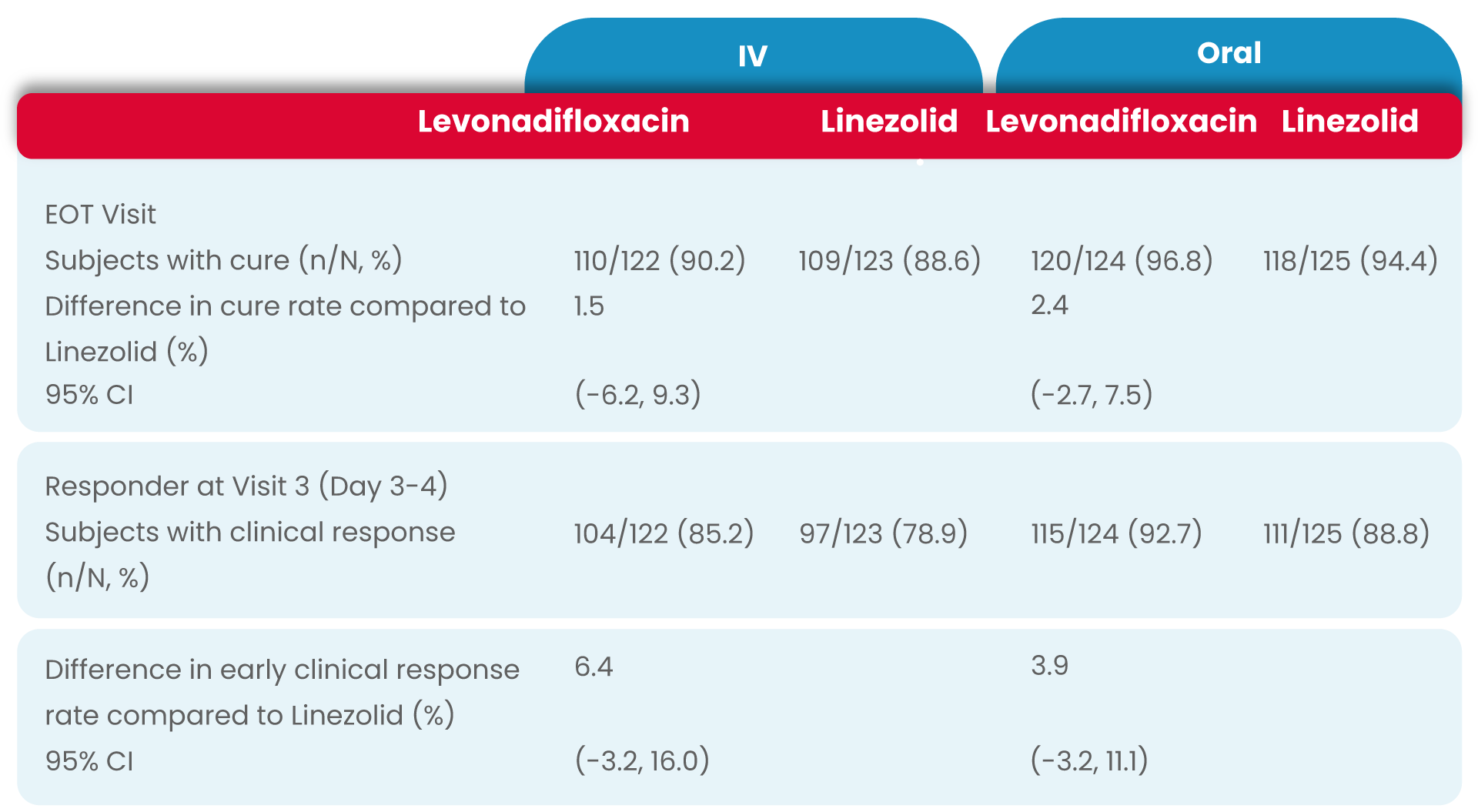
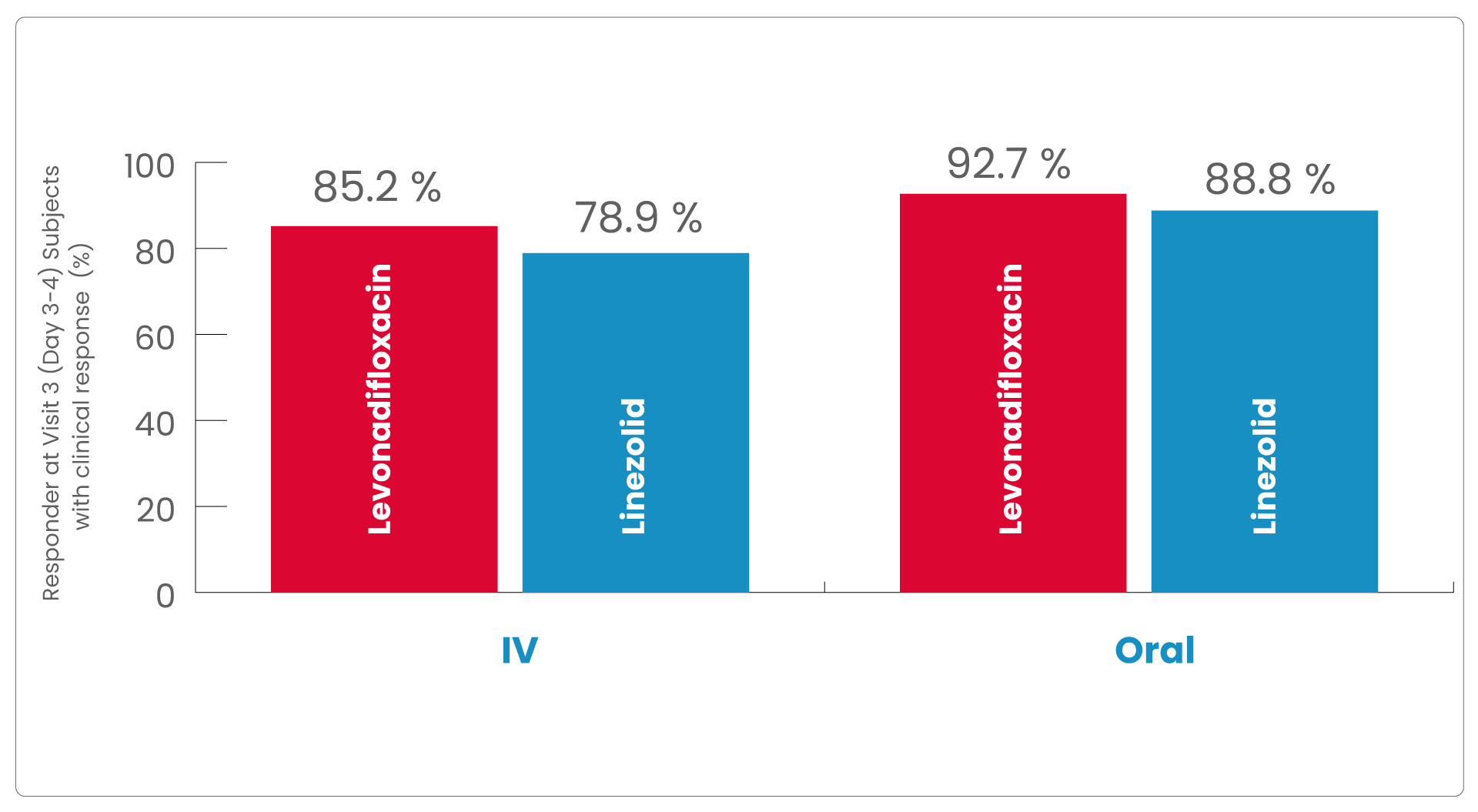
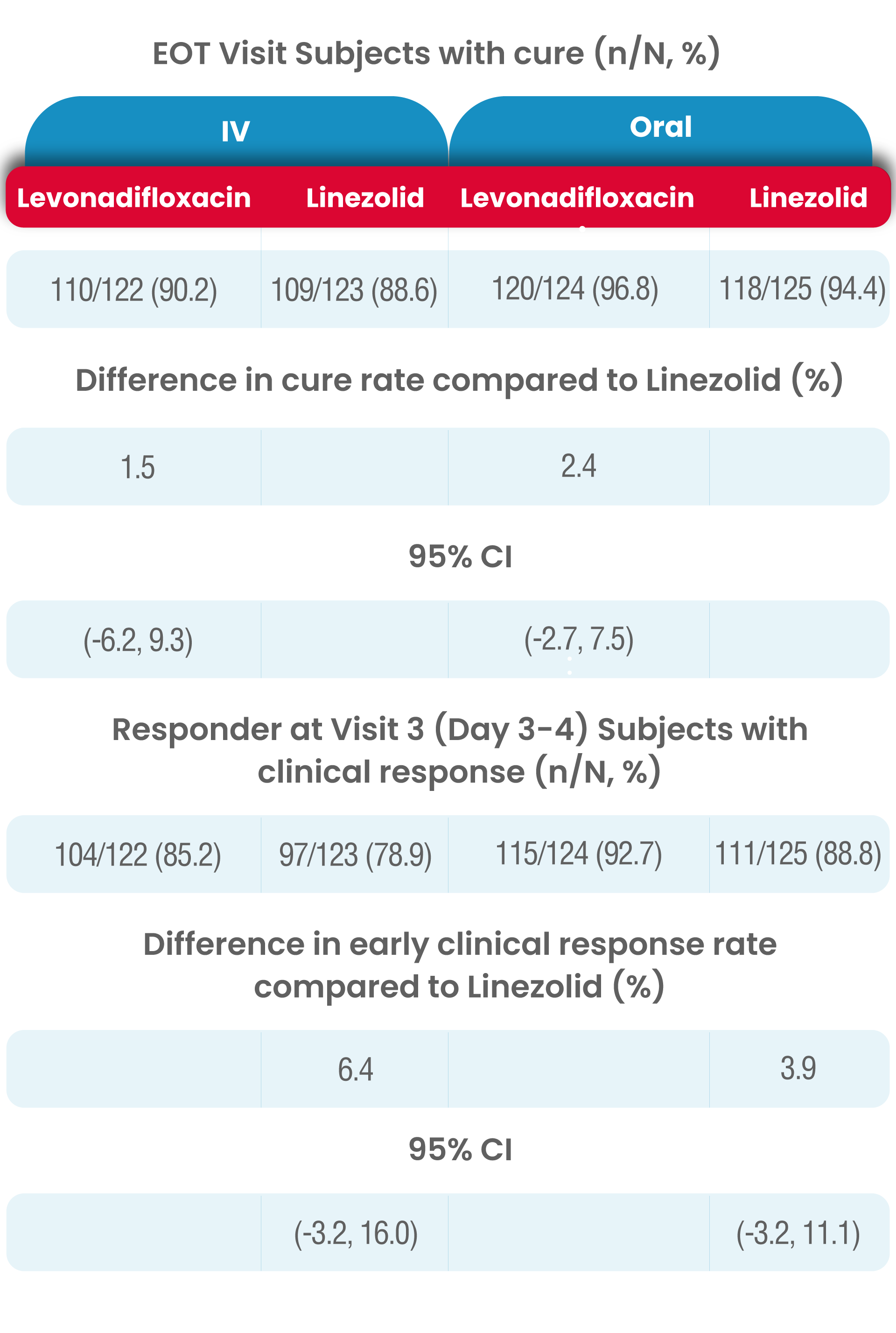

In levonadifloxacin treatment group clinical responder rate was numerically higher than linezolid treatment (85.2% vs. 78.9% in IV group and 92.7% vs. 88.8% in oral group) at Visit 3 (Day 3-4) with ≥20% reduction in lesion size compared to baseline measurement, with a treatment difference of 6.4% and 3.9% in IV and oral group, respectively.
The most common reason for subjects being classified as a non-responder was <20% reduction in lesion size.
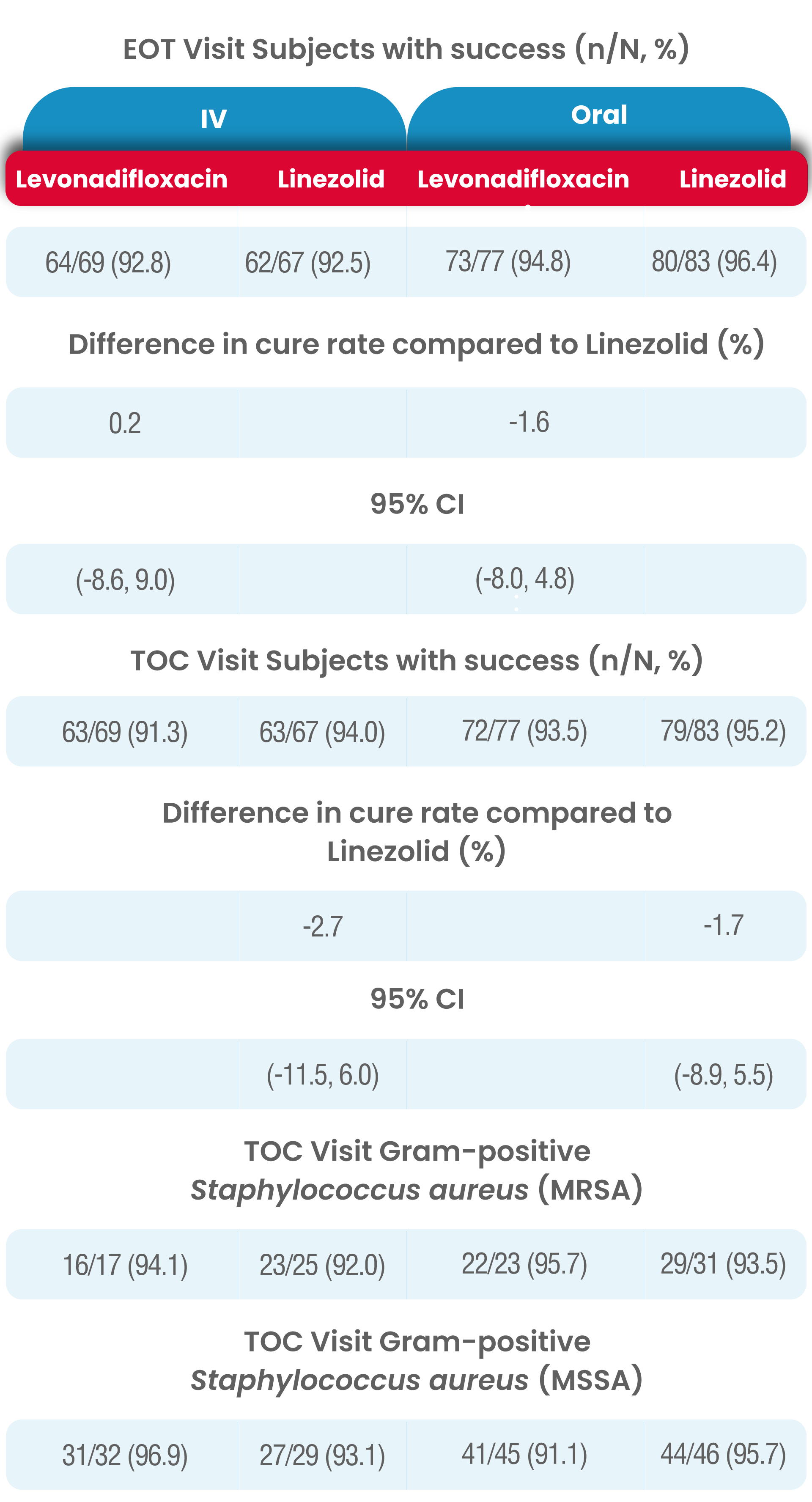
In both analysis populations, (mITT and CE-ITT), the clinical cure rates at the EOT Visit was high and comparable between the two treatment (levonadifloxacin and linezolid) groups as well as between the IV and oral subgroups.
The results of clinical response at TOC Visit in the CE-ITT population were similar to the mITT population.
Microbiological efficacy
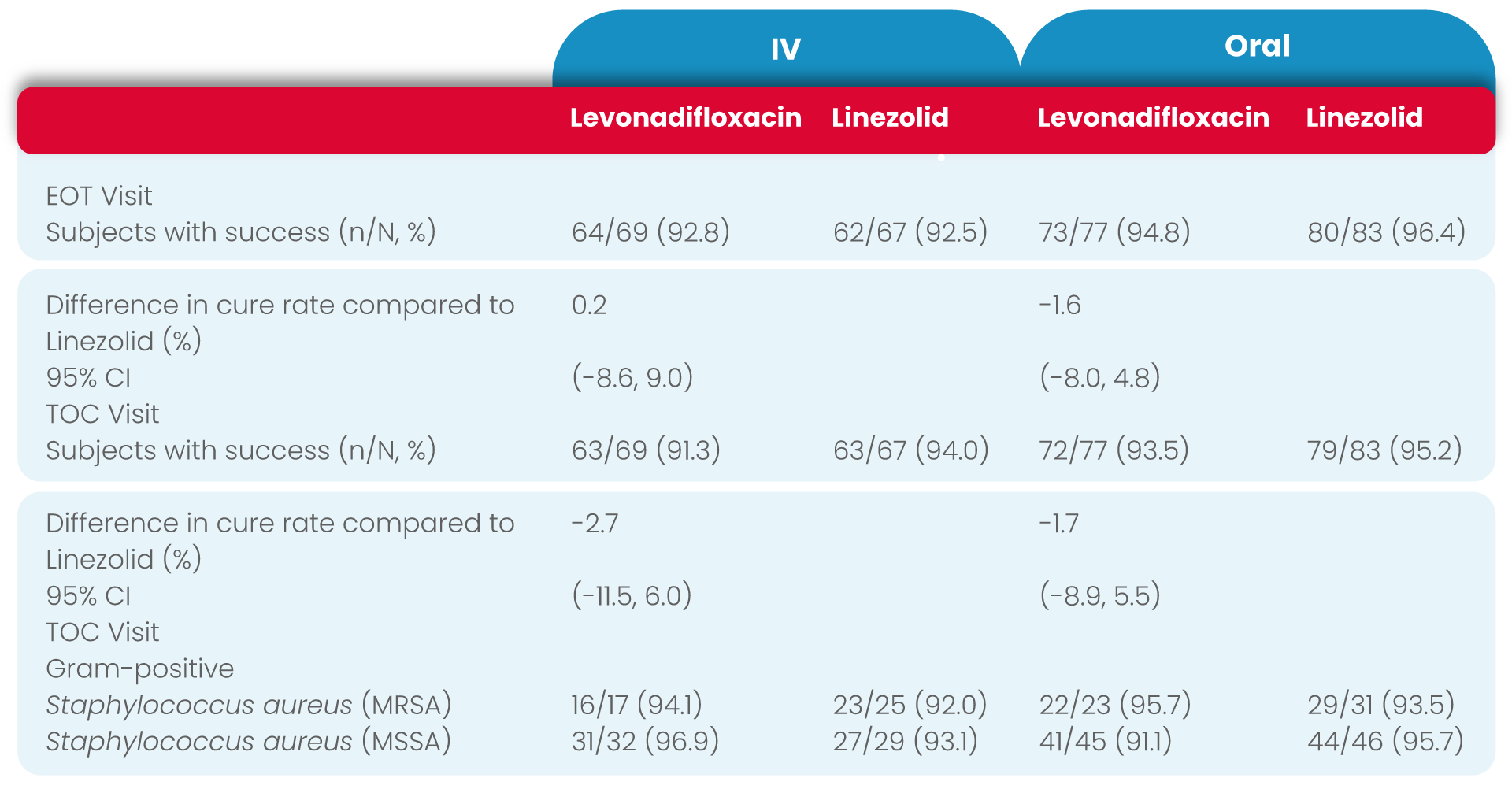
The overall results of microbiological response rates at both the EOT and TOC Visits in the ME-ITT population (IV levonadifloxacin: 98.2% and 94.5%, IV linezolid: 97.9% and 100%; oral levonadifloxacin: 97.0% and 95.5%, oral linezolid: 100% and 98.6%, respectively) was similar to the high response rates observed in the micro-ITT population (IV levonadifloxacin: 92.5% and 91.3%, IV linezolid: 92.5% and 94.0%; oral levonadifloxacin: 94.8% and 93.5%, oral linezolid: 96.4% and 95.2%, respectively).
Levonadifloxacin (IV and oral) had a higher clinical cure rate at TOC for MRSA patients compared with linezolid (IV and oral), (95.0% vs. 89.3% respectively).
Among micro-ITT subjects, four subjects in levonadifloxacin IV arm (5.8%) and one each in linezolid IV (1.5%) and oral arm (1.2%) had positive baseline blood culture, indicative of concomitant bacteraemia.
All the bacteraemia subjects in levonadifloxacin arm, exhibited favourable clinical response as well as microbiological success where as one subject in the linezolid arm was a clinical failure despite having microbiological success.
In the Diabetic Foot Ulcer sub-group, clinical cure at TOC for IV levonadifloxacin was higher than linezolid (91.7% vs 76.9%).

